What is Clinical Research?
Our patient glossary might come in handy for this article. You can read it here.
Clinical research is the study of health and illnesses in humans; and consists of clinical trials which gather data for various uses e.g. in drug development.
Unfortunately, humans have not yet evolved to live disease free; and just like all species of animals; plants and even some bacteria, we get sick. Some illnesses and conditions are much less severe than others – a common cold is preferable to the flu, and most of us would rather a short battle with a mild tension headache over an incredibly painful and debilitating cluster headache.
Since the beginning of humanity, diseases have conflicted with our desire to live as healthy social beings, surrounded by our friends and loved ones, in groups we call families.
Evidence of our desire to treat illness with drugs can be dated as far back as 3300 BC with the development of herbal medicines for various ailments.
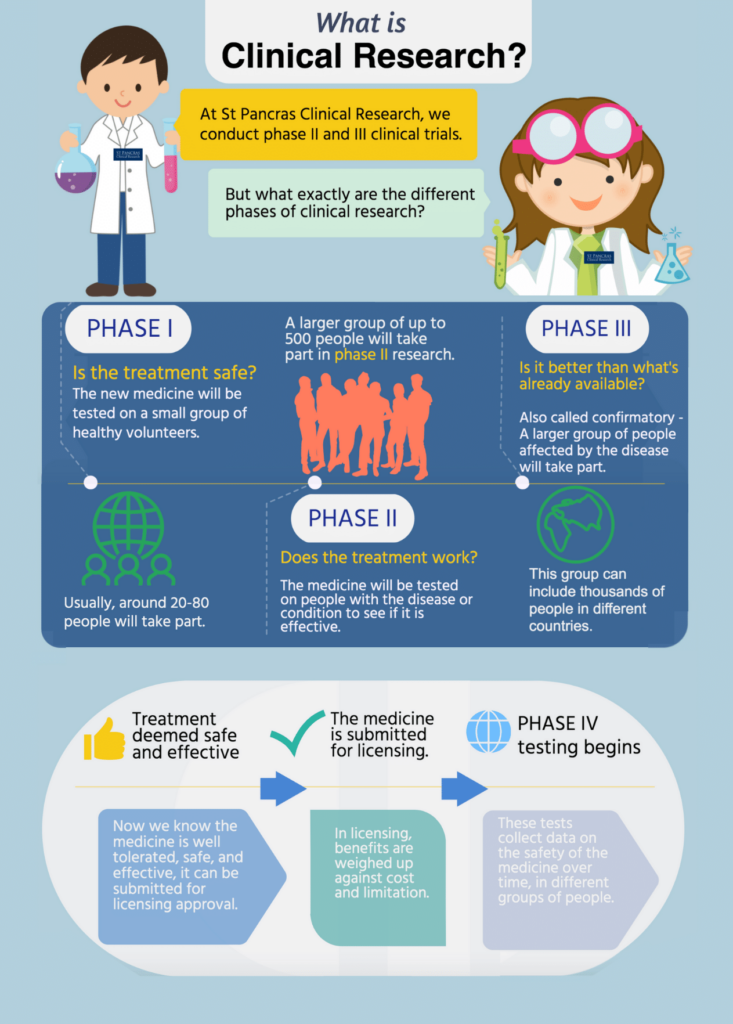
Clinical trials are the only way for new medicines to reach the public, and are the reason we have common medicines such as paracetamol, aspirin and ibuprofen. They are split usually into four different phases. Here at St Pancras Clinical Research, we conduct phase III and phase II studies.
Phase I
A phase I study is the beginning stage of testing for any new medicine in humans. They include a very small number of participants, usually around 80 healthy volunteers with no underlying health conditions.
This phase of research is done to find out whether the new medicine has any serious side effects and the safe dose of this medication. Like all phases of a clinical trial, participants are monitored very closely for the entire duration of the Phase I study.
Phase II
Phase II of a clinical trial can involve several hundred participants who are living with the illness that the medication has been designed to treat.
Researchers will monitor those enrolled in the study for several years to test the effectiveness of the medication, and to gather data on any reported side effects. Phase II studies aren’t large enough to demonstrate whether or not this new medicine is safe, which is why the study will progress to a phase III trial if everything goes to plan in phase II.
The data collected in phase II is very important for the structure and methods involved in the phase III study.
Phase III
This phase of research can involve up to 3,000 participants with the illness the medication has been designed to treat. Phase III studies can last for several years, and the aim of a phase III trial is to thoroughly evaluate how this new medication works in comparison to what is currently out there to treat the condition.
Investigators need to prove that the medicine is at the very least, as safe and as effective as what is currently available to treat the illness. If this is not proven, the drug will not be submitted for approval by the .
A process called randomization is used to select some participants to receive the new medicine, or a placebo. Sometimes instead of a placebo (a sham/dummy pill) the most current or up to date medicine is used in its place. In clinical trials testing cancer medications, placebo is very rarely used for ethical purposes.
Phase III trials are almost always double blind. This is when neither the researchers nor the participants know if they have been assigned the placebo or the investigational medicine. The reasons for this are explained here in our patient glossary, but mainly this is to help eliminate bias throughout the study.
If a phase III study has demonstrated a new medicine to be at least as safe and effective as others on the market, it will be approved.



Phase IV
After a medication has been approved, a longer phase IV study will take place over many years that involves thousands of participants. A phase IV study collects data on the long term safety of the medicine.
Clinical trials are a very important part of drug development, and allow us to know how safe and effective a new medicine is before it is made available to the general public. Most of the time, the goal of a new medicine is not to completely cure the illness itself, but to slow the progression or improve/manage the symptoms related to the illness so that the quality of life of the person with the condition is enhanced. Clinical research remains essential to ensure this can keep happening.

