We’re looking for people to take part in studies for memory loss
St Pancras Clinical Research is looking for people to take part in the development of new medicines for memory loss problems, including Alzheimer’s disease. If you are interested in taking part in one of our studies, please register your interest by clicking the button below and a member of our team will be in touch.
Your safety and well-being is our priority. We operate under the international regulations governing clinical trials, and in full partnership with your GP.
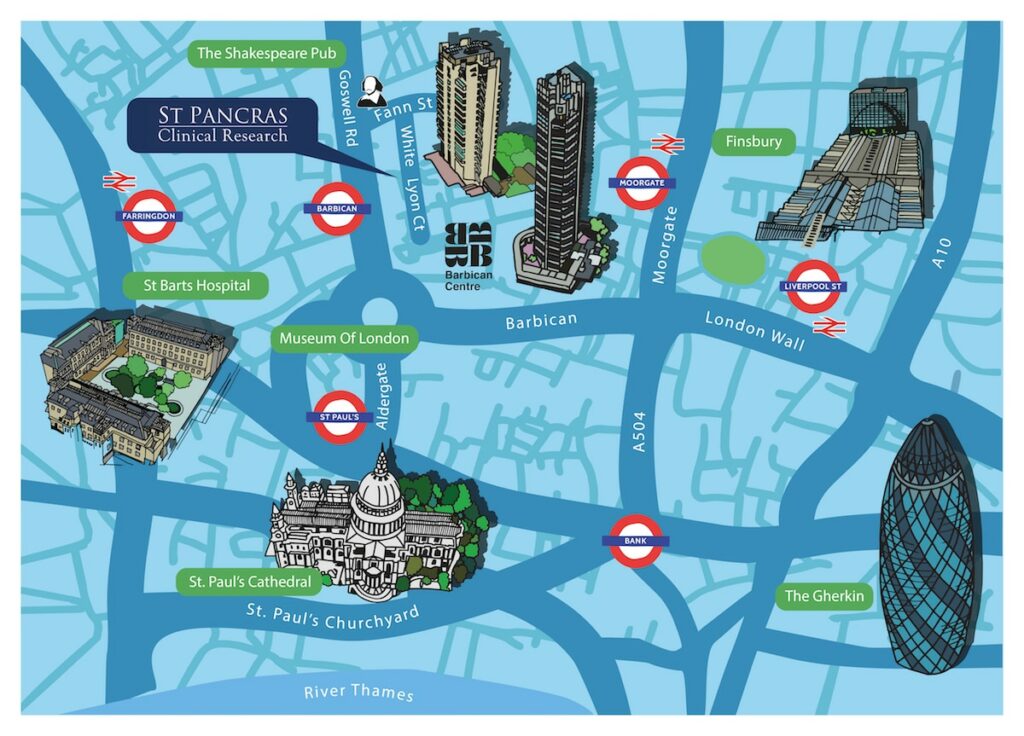
Our comfortable, friendly new clinic is located a short walk from Barbican underground station, 8 minutes from Farringdon and 12 minutes from Liverpool street.
SPCR and Memory Research
St Pancras Clinical Research was founded in 2016 by a group of medical experts with vast clinical trial experience across all phases – from ‘first-in-man’ studies, to post marketing.
SPCR has a particular interest in memory, with a team of clinicians who have over 30 years of medical and research experience in the field.
We offer the opportunity to take part in clinical trials of new medicines for the treatment of memory loss, and our patients have access to a range of specialist facilities and in-house consultants.
Our patients benefit from:
- A variety of diagnostic tests
- Early access to potential new medicines
- Access to specialist doctors and consultants
- Regular comprehensive check-ups
- Reimbursement for time and travel expenses
Am I eligible?
Please complete the form below to register your interest in our memory loss studies. All participants in a clinical trial need to meet certain criteria to take part. If you are suitable based on your completed form, a member of the team will be in touch to talk to you in more detail about your symptoms and the trial options.
Memory Studies
"*" indicates required fields